Who Does What in the Heterogeneous VTA? Spotlight on the Newly Identified NeuroD6 VTA Subtype
Material below summarizes the article The NeuroD6 Subtype of VTA Neurons Contributes to Psychostimulant Sensitization and Behavioral Reinforcement, published on May 16, 2019, in eNeuro and authored by Zisis Bimpisidis, Niclas König, Stefanos Stagkourakis, Vivien Zell, Bianca Vlcek, Sylvie Dumas, Bruno Giros, Christian Broberger, Thomas S. Hnasko, and Åsa Wallén-Mackenzie.
Highlights
- NeuroD6 VTA neurons constitute a modest ventral tegmental area (VTA) subpopulation, express Tyrosine hydroxylase (Th), and release dopamine in the nucleus accumbens upon optogenetic activation in the VTA.
- Some of the NeuroD6 neurons co-express Th with the gene encoding Vesicular glutamate transporter 2 (VGLUT2), and optogenetic VTA activation causes glutamatergic post-synaptic currents in the nucleus accumbens.
- Conditional knockout of the Vesicular monoamine transporter 2 (VMAT2) selectively in NeuroD6-Cre-neurons in mice resulted in behavioral hyperlocomotion above control levels in response to psychostimulants, while NeuroD6-Cre mice showed significant approach behavior in a real-time place preference setup upon optogenetic activation in the VTA.
 |
|
|
Study Question
We and others have recently identified molecularly distinct subpopulations of dopamine neurons within the ventral tegmental area (VTA). Our main question in the current study was: Is it possible to identify distinct behaviors that a specific VTA subpopulation contributes to? For example, what about the recently identified NeuroD6 subpopulation? Where do these neurons project, how do they signal, and what types of behaviors are they involved in?
How This Research Advances What We Know
Heterogeneity has become a central feature in many aspects of neuroscience, and this is true also for the VTA. This midbrain region was recognized in the 1960s to contain dopamine neurons, and VTA dopamine neurons have since then been shown to be involved in various aspects of reward-related behavior such as reward prediction, reinforcement, incentive salience, and motivation. Consequently, dysregulation of VTA neurons is correlated with severe brain disorders.
While it is puzzling how such a small population of cells can be involved in so many different functions, clues have appeared in the form of cellular heterogeneity. VTA dopamine neurons, long believed to comprise a rather homogeneous population, can be sorted into subpopulations/subtypes based on features such as afferent and efferent projections, electrophysiological properties, and molecular identity, properties that likely ascribe distinct neurons different functional roles. The puzzle of how distinct VTA neurons contribute to behavior is important to solve, not least to help advance drug discovery of dopamine disorders.
It has also become clear that the VTA contains GABA and glutamate-releasing neurons, as well as co-releasing neurons. These neurons affect dopamine neurons and play distinct functional roles, which means the VTA is heterogeneous is many ways.
Experimental Design or Methodology
By taking advantage of recently described gene expression patterns that distinguish dopamine neurons from each other, we reasoned it should be possible to correlate cellular identity with functional roles using the Cre-Lox transgenic system. In this system, an allele of interest to modify genetically, for example to delete or invert, is constructed to be flanked by Lox sites. Adding Lox sites, or short oligonucleotide sequences, to an allele, labels it as a target for recombination by Cre recombinase. Spatial and temporal selectivity is provided by expressing Cre under control of a promoter of interest.
In the current study, we focused on the recently identified NeuroD6 subpopulation and used mice in which Cre recombinase was expressed under control of NeuroD6 regulatory sequences, called NEX-Cre mice. We also included Dopamine transporter (DAT)-Cre mice to enable the comparison between NeuroD6 VTA neurons and the majority of VTA dopamine neurons in behavioral regulation.
We used two different Cre-Lox systems for functional analysis: first, conditional genetics to abrogate the ability for NeuroD6 VTA neurons to signal with dopamine, and second, optogenetics to drive NeuroD6 VTA neuronal activation in real-time. We also used optogenetics to study the neurocircuitry of NeuroD6 VTA neurons and to validate neurotransmitter release upon stimulation.
In addition to NeuroD6-Cre and DAT-Cre mice, we included VGLUT2-Cre mice and Calbindin2 (Calretinin)-Cre mice for validation: VGLUT2-Cre to address glutamatergic VTA neurons as control, and Calbindin2 (Calb2)-Cre based on our previous results showing these represent a dopamine subpopulation that was of interest to compare with the NeuroD6 VTA population.
Results
We had previously shown NeuroD6 mRNA can be found primarily in the medioventral part of the VTA, while it is almost entirely excluded from other parts of the VTA and the adjacently located substantia nigra.
First, we first asked if all NeuroD6 neurons in the VTA were dopamine neurons. By a co-labeling approach using in situ hybridization for NeuroD6 mRNA and Th mRNA, which encodes for the rate-limiting enzyme of dopamine synthesis, we found that all NeuroD6-positive VTA neurons were positive for Th mRNA.
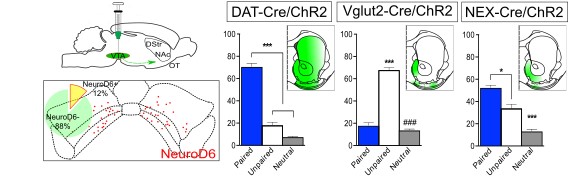
Next, we asked what proportion of NeuroD6 dopamine neurons were among the whole VTA dopamine population, and found that 12% of VTA dopamine neurons were positive for NeuroD6.
Then, because the VTA dopamine neuron population contains GABA and glutamate co-releasing neurons, we addressed markers for GABA or glutamate. We identified that less than 15% of the NeuroD6/Th neurons were positive also for Vglut2 mRNA. This provided a molecular basis for our next finding, namely that optogenetic activation of NeuroD6 VTA neurons, in NEX-Cre mice expressing Channelrhodopsin2, resulted in dopamine release and glutamatergic post-synaptic currents in the nucleus accumbens shell.
To see if we could identify any correlation between altered activity of NeuroD6 VTA neurons and behavioral output, we first generated Vmat2lox/lox;NEX-Cre conditional knock-out mice. In these mice, Cre recombinase inactivates the gene encoding Vesicular monoamine transporter 2 in NeuroD6 VTA neurons so they cannot release dopamine from synaptic vesicles. These Vmat2lox/lox;NEX-Cre conditional knock-out mice showed a hyperlocomotive response upon injection with either amphetamine or cocaine, which was stronger than the psychostimulant-induced locomotion shown by control mice. Dopamine signaling from NeuroD6 VTA neurons thus seems to be required for normal locomotor response upon psychostimulant injection.
While conditional genetics allowed the analysis of NeuroD6 neurons in behavior, this method can give rise to phenotypes that are due to developmental adaptations. Therefore, to address a direct correlation between stimulation of NeuroD6 VTA neurons in the adult mouse and behavioral activation, we implemented optogenetics with optical fibers implanted above the VTA. NEX-Cre mice expressing ChR2 in the VTA were compared with DAT-Cre, VGLUT2-Cre and Calb2-Cre mice, all expressing ChR2 in the VTA.
We confirmed DAT-Cre mice showed place preference and VGLUT2-Cre mice place avoidance upon optogenetic activation in the VTA. Calb2-Cre mice showed no preference at all, but NeuroD6-Cre mice showed a place preference behavior. This response was potentiated when we used bilateral rather than unilateral light stimulation. These results identified a direct correlation beween activation of VTA NeuroD6 neurons and place preference behavior. It also showed NeuroD6 and Calbindin2 VTA neurons have distinct behavioral profiles.
Interpretation
Aberrant signaling by VTA dopamine neurons is strongly implicated in affective and cognitive dysfunction. Consequently, several neuropsychiatric disorders, including ADHD and schizophrenia, are treated with medication aimed to correct dopamine function. However, no current medication aimed at the dopamine system can selectively affect only those neurons that cause symptoms and leave healthy neurons intact. By uncovering which VTA DA neurons contribute to various aspects of behavioral regulation, the research field aims toward increased knowledge of the correlation between distinct neurons and their role in behavior, and ultimately toward the possibility of using this increased knowledge to enable selective treatment of complex disorders.
Our study contributes to this research and enabled the correlation of NeuroD6 VTA neurons with psychostimulant-induced locomotion and certain aspects of approach behavior. Moving forward, recently described intersectional methodology, in which combinations of markers can be used to identify the neurocircuitry and functional role of VTA subtypes, will likely offer new exciting opportunities to advance the puzzle of “Who does what in the VTA?”
Visit eNeuro to read the original article and explore other content. Read other summaries of eNeuro and JNeurosci papers in the Neuronline collection SfN Journals: Research Article Summaries.
The NeuroD6 Subtype of VTA Neurons Contributes to Psychostimulant Sensitization and Behavioral Reinforcement. Zisis Bimpisidis, Niclas König, Stefanos Stagkourakis, Vivien Zell, Bianca Vlcek, Sylvie Dumas, Bruno Giros, Christian Broberger, Thomas S. Hnasko, and Åsa Wallén-Mackenzie. eNeuro May 2019, 6 (3) 0066–19.2019; DOI: 10.1523/ENEURO.0066-19.2019





