The Shape of Brain Waves Recorded From the Scalp Differentiates Parkinson's Disease
Material below summarizes the article Characteristics of Waveform Shape in Parkinson’s Disease Detected with Scalp Electroencephalography, published on May 20, 2019, in eNeuro and authored by Nicko Jackson, Scott R. Cole, Bradley Voytek, and Nicole C. Swann.
Highlights
- We show that the shape of brain waves recorded over sensorimotor regions in patients with Parkinson’s disease (PD) was more asymmetric in patients off medication than in patients on medication and in healthy controls.
- We detected these patterns using a safe, affordable, and accessible recording method: scalp EEG (electroencephalography, brain recordings taken from the scalp).
- An objective measure of PD like this could be used in the future for diagnosis, monitoring of disease progression, or adjusting treatments.
 |
|
|
Study Question
We used a novel approach for analyzing neural signals (“brain waves”) to detect signatures of Parkinson’s disease from brain recordings acquired from the scalp (taken using electroencephalography, or EEG). This novel approach quantifies asymmetries in the shape of the brain waves. For example, is the peak sharper (or “pointier”) than the trough?
How This Research Advances What We Know
Currently, Parkinson’s disease (PD) is mainly diagnosed and monitored using clinical rating scales. These measures are subjective and can be imprecise. Thus, an objective measure of PD is needed. Electrical brain recordings represent one possible objective measure of PD.
Previous research has shown that PD is marked by overly synchronized brain activity. In humans, this phenomenon has predominantly been demonstrated with recordings from the basal ganglia acquired during neurosurgery. In these studies, conventional measures of synchronized brain activity, like the power of oscillatory activity, relate to PD symptoms.
Detecting these signatures using a safe and noninvasive method, like scalp EEG, would improve the clinical utility. Unfortunately, EEG power doesn’t reliably distinguish PD. Recently, novel measures of synchronized activity that quantify oscillation shape have been introduced. These have shown promise for distinguishing PD in neurosurgical recordings. Here we tested if waveform shape detected with EEG could also distinguish PD.
Experimental Design or Methodology
We analyzed a previously published EEG dataset, acquired with a 32-electrode recording system, from 15 PD patients off and on their PD medications and 16 healthy participants. We focused our main analysis on the electrodes closest to motor cortex (C3 and C4).
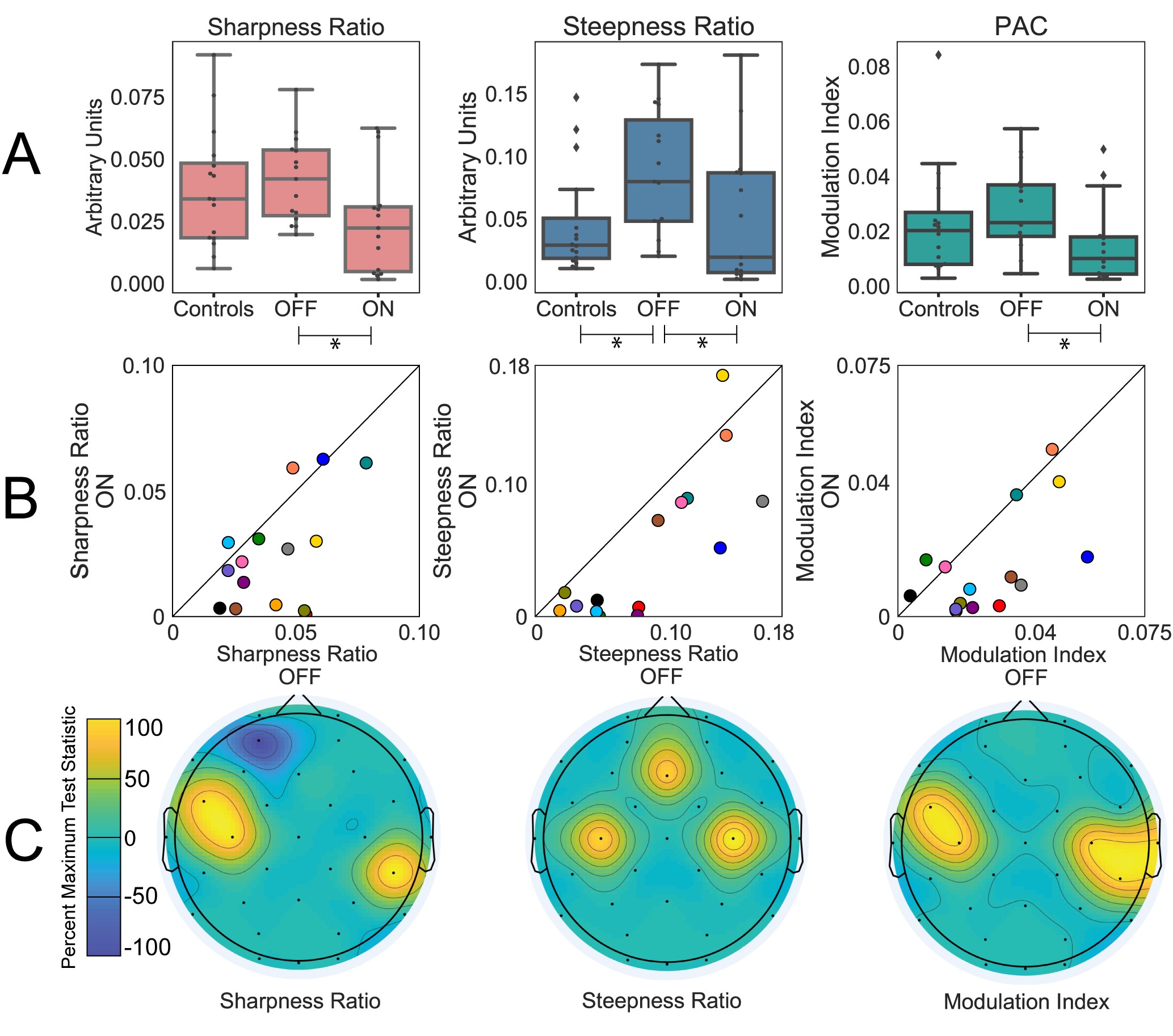
We quantified waveform shape as both the sharpness ratio and steepness ratio. These measures reflect the asymmetry of the waveform shape. Sharpness ratio reflects how “sharp” (or pointy) the peak of a waveform is compared to the trough. Steepness compares the maximum slope of the rise of a waveform to its fall.
For both these measures we used nonparametric statistics to compare groups (Wilcoxon signed rank for patients on versus off medication and Wilcoxon rank sum for patients to controls). A false discovery rate correction was used for multiple comparisons. Effect size (Cohen’s d) was calculated and reported for each of the measures described.
Results
Focusing first on the electrodes closest to sensorimotor cortex, we found that waveform shape was more asymmetric in patients with Parkinson’s disease off medications compared to on. This was apparent in significant decreases of both sharpness ratio and steepness ratio with medication and was accompanied by a clinical improvement in symptoms.
To examine the scalp topography more broadly, we repeated the same on versus off medication comparison for the rest of the electrodes and found that results were consistent with a sensorimotor origin. Specifically, we assessed if muscle artifact may have influenced our findings by comparing the results for electrodes closest to the temporalis muscle (near the temples) and found no significant difference for the on versus off medication comparison.
Waveform shape, specifically the steepness ratio measure, also differentiated the PD patients off medication from the healthy control group. However, sharpness ratio did differentiate these groups.
Using these waveform shape measures we were also able to quantify the overall shape of the dominant waveforms over sensorimotor areas. We found this shape was characterized by a sharper peak and a steeper decay, suggesting a canonical arched shape. This shape was not present for other electrodes, specifically those likely to be impacted by muscle artifacts. These findings motivate the idea of focusing future analyses on portions of data with this particular waveform shape, since these portions of data are more likely to contain a dominate sensorimotor oscillation.
Interpretation
Examining waveform shape may provide insight into underlying neural interactions. For instance, modeling work has suggested that sharper waveforms may reflect hypersynchronous inputs, in this case perhaps from the basal ganglia to cortex. The excessive synchrony seen in the motor systems of patients with PD may constrain neurons in a fixed firing pattern, which could prevent dynamic changes necessary for normal motoric behavior. Dopaminergic medication reduces this synchrony, which is reflected in the changes in waveform shape.
Here we showed that waveform shape, a novel way to quantify electrophysiological activity, reflects PD state and can be detected with EEG. Not only does this motivate consideration of waveform shape in future EEG studies, but it also suggests that waveform shape may be a putative noninvasive biomarker of PD. An objective measure like this could aid in diagnosis, monitoring for disease progression, and signaling when a treatment regimen may need adjustment.
Visit eNeuro to read the original article and explore other content. Read other summaries of eNeuro and JNeurosci papers in the Neuronline collection SfN Journals: Research Article Summaries.
Characteristics of Waveform Shape in Parkinson’s Disease Detected with Scalp Electroencephalography. Nicko Jackson, Scott R. Cole, Bradley Voytek, and Nicole C. Swann. eNeuro May 2019, 6 (3) 0151–19.2019; DOI: 10.1523/ENEURO.0151-19.2019







