Material below summarizes the article 3D Imaging of Axons in Transparent Spinal Cords from Rodents and Nonhuman Primates, published on March 26, 2015, in eNeuro and authored by Cynthia Soderblom, Do-Hun Lee, Abdul Dawood, Melissa Carballosa, Andrea Jimena Santamaria, Francisco D. Benavides, Stanislava Jergova, Robert M. Grumbles, Christine K. Thomas, Kevin K. Park, James David Guest, Vance P. Lemmon, Jae K. Lee, and Pantelis Tsoulfas.
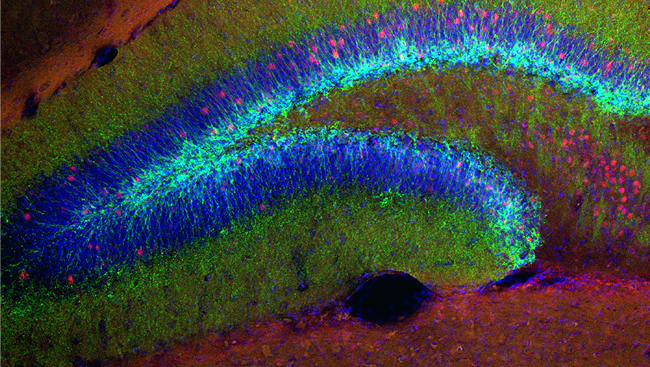
The beginning of this century has seen some major advances in light microscopy, particularly related to neuroscience. These developments in microscopy, coupled with techniques that make tissues transparent, are enabling microscopes to visualize the cellular architecture of whole tissues in 3D with unprecedented detail.
One of these advances in microscopy has been light sheet fluorescent microscopy (LSFM). The underlying method was developed in 1902 by Richard Zsigmondy and Henry Siedentopf to enhance the microscopic resolution for studying colloidal gold. The modern light sheet fluorescence microscopy was first pioneered by Voie and colleagues and originally named orthogonal-plane fluorescence optical sectioning. Arne Voie, David Burns, and Francis Spelman focused a laser beam into a thin sheet to illuminate a fluorescent sample and captured the reflected light using a different objective lens-oriented perpendicular to the plane of illumination (i.e., light sheet).
This is in contrast to confocal laser microscopy, where the laser and the reflected light travel through the same objective lens. Using this method, these authors were able to reconstruct a cleared guinea pig cochlea in 3D. The basic configuration of that instrument lay the foundation for all subsequent versions of LSFM microscopes.
In confocal microscopes, the optical sectioning of the specimen is based on discriminating the out-of-focus reflected light by using a pinhole. However, the excitation light excites all of the fluorophores as it passes though the specimen, which often leads to photobleaching. Furthermore, point-by-point scanning along the entire specimen makes imaging too slow and unwieldy for large specimens that are millimeters in thickness. In addition, there is a drop in image brightness with increasing depth caused by light scattering and absorption. In LSFM, the laser light sheet, typically 2-6 microns, illuminates only one thin plane of the sample surrounding the focal plane of the detection lens, and thus there is no out of focus light. Therefore, the photo-bleaching or photo-damage is much less than conventional laser scanning microscopy including 2-photon systems.
One limitation of LSFMs using single side illumination, especially with large tissue, is that any obstacles (e.g., air bubbles or high concentration of fluorophores) cast shadows along the illumination path, which appear as stripes in the acquired image. A way to get around this problem is to illuminate from two opposing sides and merge the images, such as in the Ultramicroscope. In addition, adjusting the thickness of the laser sheet and the use of multi-view imaging can provide enhanced resolution. Thus, LSFM is ideal for 3D reconstruction of clear specimens and for in vivo imaging of transparent organisms.
Similar to light microscopy, there have been significant advances in tissue-clearing techniques during this century.
The first successful attempt to render fixed anatomical preparations transparent was done by Walter Spalteholtz. He used a solution of benzyl alcohol and methyl salicylate, which was later modified by others to produce Murray’s clear solution. Murray's clear was mostly used to study development of vertebrate embryos.
The first 3D reconstructions of clear embryos were obtained by using optical projection tomography, and later, Hans-Ulrich Dodt’s lab pioneered the use of light sheet microscopy on cleared whole-mount specimens, such as whole mouse brains expressing green fluorescent protein (GFP).
Dodt and Frank Bradke’s laboratories later developed the 3DISCO (3-dimensional imaging of solvent cleared organs) method of imaging rodent brain and spinal cord by combining Ultramicroscopy with tetrahydrofuran-based tissue clearing.
A few years later, Karl Deisseroth’s lab developed CLARITY, which is a hydrogel-based method that also allows antibody penetration for immunohistochemical labeling in whole tissue. CLARITY is also compatible with LSFM, a method termed COLM (clarity-optimized light-sheet microscopy). Both 3DISCO and CLARITY have relied on the use of transgenic mice in which subpopulation of neurons are brightly labeled with GFP.
However, we have developed AAV (adeno-associated virus)-based fluorescent labeling methods that can be used with 3DISCO to image non-transgenic animals such as rats. In addition, the use of viruses allows much better spatiotemporal control over labeling methods as compared to transgenic animals. Therefore, the combination of LSFM with multiple tissue clearing strategies and neuronal labeling methods will greatly aid our understanding of the structure-function relationship of the central nervous system under normal and diseased conditions.
Visit eNeuro to read the original article and explore other content. Read other summaries of JNeurosci and eNeuro papers in the Neuronline collection SfN Journals: Research Article Summaries.
3D Imaging of Axons in Transparent Spinal Cords from Rodents and Nonhuman Primates. Cynthia Soderblom, Do-Hun Lee, Abdul Dawood, Melissa Carballosa, Andrea Jimena Santamaria, Francisco D. Benavides, Stanislava Jergova, Robert M. Grumbles, Christine K. Thomas, Kevin K. Park, James David Guest, Vance P. Lemmon, Jae K. Lee, Pantelis Tsoulfas. eNeuro Mar 2015, 2 (2) DOI: 10.1523/ENEURO.0001-15.2015