Morphine Reduces Neuronal Connectivity by Releasing Iron From Storage
Material below summarizes the article Morphine-Induced Modulation of Endolysosomal Iron Mediates Upregulation of Ferritin Heavy Chain in Cortical Neurons, published on July 12, 2019, in eNeuro and authored by Bradley Nash, Kevin Tarn, Elena Irollo, Jared Luchetta, Lindsay Festa, Peter Halcrow, Gaurav Datta, Jonathan D. Geiger, and Olimpia Meucci.
Highlights
- Morphine exposure increases free iron levels in neurons through the release of iron from intracellular storage sites called endolysosomes.
- Increased free iron in the cytosol causes neurons to produce additional ferritin heavy chain, a protein involved in iron storage that can also reduce neuronal connectivity by inhibiting a homeostatic process.
- This pathway may help to explain why opioid-using people infected with human immunodeficiency virus (HIV) are more likely to develop learning and memory problems and suggests that these problems may be treatable by targeting neuronal iron stores.
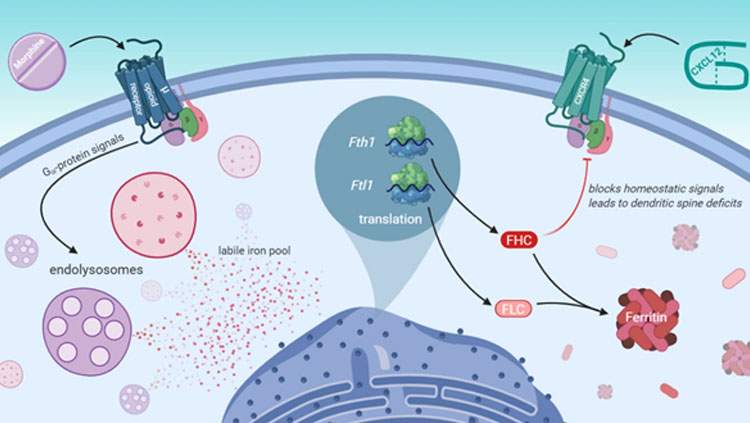
Morphine activates a µ-opioid receptor G-protein signaling pathway that leads to efflux of iron stored in endolysosomes. In response, cortical neurons produce additional ferritin heavy chain, which is a protein involved in iron storage that also contributes to dendritic spine deficits by inhibiting the homeostatic chemokine receptor CXCR4. (Nash et al. eNeuro 2019, inspired by Figure 8).
Study Question
Evidence suggests that morphine and other kinds of licit and illicit opioids contribute to learning and memory problems in HIV patients by reducing neuronal connectivity in specific brain areas. Therefore, our main question was: What are the molecular factors that lead to reduced neuronal connectivity after exposure to morphine?
How This Research Advances What We Know
Despite widespread use and benefits of HIV therapies, people with HIV still develop learning and memory problems as they age. These problems are worsened by opioid use, which is very common among people infected with HIV.
Opioids and HIV infection can cause subtle functional changes and reduced connectivity of neurons in specific brain areas, which is thought to contribute to cognitive symptoms. However, the means by which opioid use contributes to these subtle neuronal changes is not completely understood.
Previously, we showed that µ-opioid compounds like morphine increased the levels of an iron storage protein called ferritin heavy chain in cortical neurons, which was required for morphine to reduce neuronal connectivity.
This new study showed that morphine released iron stored in intracellular compartments called endolysosomes, and that this pool of iron was required for morphine to increase ferritin heavy chain levels and reduce the connectivity of cortical neurons.
Experimental Design or Methodology
We performed most experiments in this study using primary cultures of rat cortical neurons and used standard approaches to measure the levels of select proteins and messenger RNAs. We measured the number of connection sites among neurons using microscopy with fluorescent dyes that label dendritic spines, the post-synaptic structures of excitatory synapses. To measure iron levels in sub-cellular compartments, we allowed neurons to take up fluorescent iron indicators that naturally reside in either endolysosomes or the cytoplasm and measured their brightness. We repeated each experiment at least three times, each time using neurons from a different litter of rats.
We also determined if certain results from our neuronal cultures translated to the rat brain by staining brain tissue sections from morphine-treated rat pups. To reduce experimenter bias, lab members who imaged and analyzed these brain sections did not know if they were from placebo or morphine-treated rats.
Results
From previous research, we knew that 24-hour exposure to morphine increased ferritin heavy chain levels by activating the µ-opioid receptor, which is a protein that transmits opioid signals into cells. Here, we found that a specific type of µ-opioid receptor signal through G-proteins led to ferritin heavy chain production. Interestingly, this µ-opioid receptor G-protein signal only increased ferritin heavy chain protein levels in the cytoplasm and did not change the amount of its messenger RNA.
When morphine increased ferritin heavy chain levels, we also observed a reduction of specific types of dendritic spines that are thought to underlie short-term (learning) and long-term (memory) processes. This spine reduction was also prevented by drugs that block signals from the µ-opioid receptor or its corresponding G-protein.
Furthermore, morphine-treated rats showed increased neuronal ferritin heavy chain levels and a reduction of the same dendritic spine types in the medial prefrontal cortex, a critically important brain area for learning and memory processing.
We thought morphine might increase ferritin heavy chain levels by increasing neuronal iron levels, as iron can increase the translation of ferritin heavy chain messenger RNAs. Interestingly, morphine decreased endolysosomal iron levels after 30 minutes and increased cytoplasmic free iron levels out to at least 24 hours. Chelation of extracellular iron did not alter morphine’s effects, but strikingly, selective chelation of endolysosomal iron completely prevented morphine’s ability to increase ferritin heavy chain levels and reduce dendritic spines in cortical neurons.
Interpretation
Our results suggest that opioids’ ability to increase ferritin heavy chain levels and reduce neuronal connectivity requires iron stored in endolysosomes. Therefore, new therapeutic approaches targeting endolysosomal iron or opioids’ ability to liberate iron from these compartments could restore neuronal connectivity and alleviate learning and memory problems in opioid using HIV patients.
Our current research is focused on better understanding this process, including which downstream opioid signals and endolysosomal channels or transporters are involved in iron release, and how morphine affects the production of other iron-related proteins that might contribute to learning and memory problems.
Our findings may also be broadly relevant to other brain diseases where neuronal iron levels are increased, like Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS). Although these diseases may increase neuronal iron levels in a different way than morphine, iron may still be partly responsible for cognitive symptoms in these disorders.
Visit eNeuro to read the original article and explore other content. Read other summaries of eNeuro and JNeurosci papers in the Neuronline collection SfN Journals: Research Article Summaries.
Morphine-Induced Modulation of Endolysosomal Iron Mediates Upregulation of Ferritin Heavy Chain in Cortical Neurons. Bradley Nash, Kevin Tarn, Elena Irollo, Jared Luchetta, Lindsay Festa, Peter Halcrow, Gaurav Datta, Jonathan D. Geiger, and Olimpia Meucci. eNeuro 12 July 2019, 6 (4) 0237-19.2019; DOI: 10.1523/ENEURO.0237-19.2019.







