Lowering Prepubertal GnRH Neuron Activity Changes Adult Activity and Reproductive Cycles
Material below summarizes the article Chemogenetic Suppression of GnRH Neurons during Pubertal Development Can Alter Adult GnRH Neuron Firing Rate and Reproductive Parameters in Female Mice, published on June 8, 2020, in eNeuro and authored by Eden A. Dulka, R. Anthony DeFazio, and Suzanne M. Moenter.
Highlights
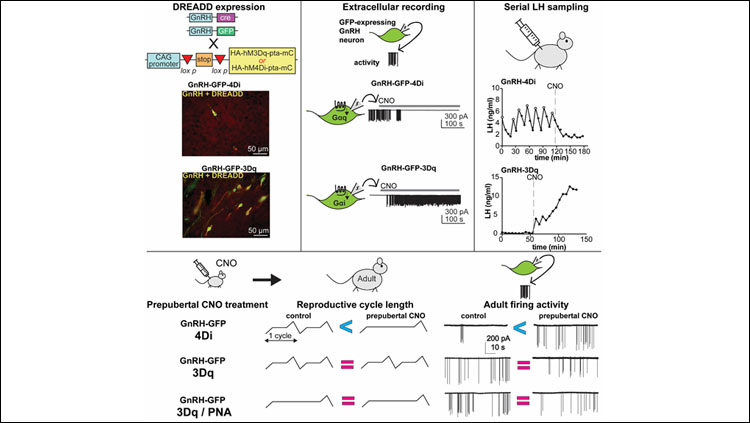
- Chemogenetic manipulation of Gonadotropin-releasing hormone (GnRH) neuron firing rate results in the expected changes in GnRH neuron firing and pituitary hormone release.
- Suppressing prepubertal GnRH neuron activity leads to increased adult GnRH neuron firing rate and days in diestrus.
- Activating prepubertal GnRH neuron activity had no effect on adult firing rate and did not rescue adult reproductive phenotypes in mice exposed to androgens before birth.
Study Question
Do changes in prepubertal GnRH neuron activity help establish the adult GnRH neuronal network and adult reproductive function? Can correcting altered prepubertal GnRH neuron firing in a mouse model of reproductive dysfunction ameliorate disruptions in adult reproductive function and GnRH neuron firing?
How This Research Advances What We Know
Gonadotropin-releasing hormone (GnRH) neurons link the brain to the downstream reproductive system via GnRH release. GnRH regulates luteinizing hormone (LH) secretion from the pituitary; LH influences gametogenesis and steroidogenesis in the gonad. Persistent high frequency LH, and presumably GnRH, release is a hallmark of polycystic ovary syndrome (PCOS), the leading cause of female infertility. Clinical evidence suggests characteristics of PCOS emerge prior to puberty. Prenatally androgenized (PNA) animal models, which recapitulate many aspects of PCOS, have noted upregulation of the adult reproductive neuroendocrine axis including increased LH release frequency. In female mice, PNA treatment reduces prepubertal but elevates adult GnRH neuron activity. Given that PNA alters prepubertal GnRH neuron activity and similar early-life activity helps organize other neuronal networks and physiologic output, we sought to examine the role of prepubertal GnRH neuron activity in adult reproductive function and to determine if reversing PNA-induced prepubertal decreases in neuronal activity were sufficient to ameliorate adult reproductive dysfunction.
Experimental Design or Methodology
To determine if altering prepubertal GnRH neuron activity influences adult neuronal activity and/or reproductive cycles on its own without PNA treatment, we expressed the excitatory (3Dq) or inhibitory (4Di) designer receptors exclusively activated by designer drugs (DREADDs) specifically in GnRH neurons. DREADDS can change neuronal activity upon binding of the chemical clozapine-N-oxide (CNO). We verified correct expression and confirmed CNO mediated activation and inhibition of GnRH neurons via electrophysiological recordings of GnRH neurons in brain slices and serial sampling of LH release in vivo. CNO was administered to control or PNA mice between two to three weeks of age. Mice were subsequently monitored for pubertal timing (vaginal opening and first estrus), and reproductive cyclicity. Recordings of firing rate and GABA transmission were also performed in adulthood. Data distributions were tested using a Shapiro-Wilk normality test and statistical analyses were selected based upon experimental design and data distribution.
Results
These studies investigated the role of prepubertal GnRH neuron activity in setting up the adult GnRH network and adult reproductive function. Control experiments demonstrated that DREADDs were correctly targeted to GnRH neurons and that the expected changes in neuronal output (assayed via firing rate) and modulation of the reproductive axis (assayed via LH output) were achieved. Neither suppression nor activation of GnRH neurons during the prepubertal period altered time of vaginal opening or first estrous. Suppression of prepubertal GnRH neuron activity, via the 4Di DREADD, resulted in a mild lengthening of reproductive cycles and a significant increase in adult GnRH neuron firing rate. This prepubertal reduction in firing rate however did not yield a change in GABAergic transmission to these cells. In both control and PNA mice in which prepubertal GnRH neuron activity was increased via the 3Dq DREADD, no significant changes in cyclicity or adult GnRH neuron firing rate were observed. A caveat is that 3Dq DREADD expression without CNO elevated GnRH neuron activity, as has been reported in other systems. Results with 3Dq thus need to be interpreted with caution.
Interpretation
These studies, the first to express DREADDs in GnRH neurons to alter their activity, demonstrate prepubertal GnRH neuron activity can influence the adult GnRH neuron network and reproductive function. Results from suppressing prepubertal GnRH neuron activity support that reducing early firing of these neurons in the absence of PNA exposure can influence adult neuronal activity and reproductive cycles, but androgens are needed for expression of the PNA phenotype such as early puberty and altered GABAergic transmission to GnRH neurons in adults. These findings are also consistent with previous observations in PNA mice in which decreased prepubertal GnRH neuron activity is associated with elevated activity and disrupted cyclicity in adulthood. Overall these studies increase our understanding of how prepubertal GnRH neuron activity influences elements of adult reproductive function and indicate that androgen-induced programming of factors beyond GnRH activity is required for full reproductive phenotypes observed in PNA models and perhaps PCOS.
Visit eNeuro to read the original article and explore other content. Read other summaries of eNeuro and JNeurosci papers in the Neuronline collection SfN Journals: Research Article Summaries.
Distinct Genetic Signatures of Cortical and Subcortical Regions Associated with Human Memory. Pin Kwang Tan, Egor Ananyev, and Po-Jang Hsieh. eNeuro 9 December 2019, 6 (6) 0283-19.2019; DOI: 10.1523/ENEURO.0283-19.2019.






