Estrogen Benefits α-Synuclein Tetramerization and Motor Deficit in PD-Like Mice
Material below summarizes the article Female Sex and Brain-Selective Estrogen Benefit α-Synuclein Tetramerization and the PD-like Motor Syndrome in 3K Transgenic Mice, published on September 18, 2019, in JNeurosci and authored by Molly M. Rajsombath, Alice Y. Nam, Maria Ericsson, and Silke Nuber.
Highlights
- Female sex preserves physiological a-synuclein (αS) tetramer formation vs. monomer clustering into vesicle and lipid-rich aggregates.
- Increasing brain estrogen improves αS tetramer-to-monomer ratio, neurite fiber abundance and mitigates the Parkinson’s disease-like motor deficits.
- Female sex, and estrogen in particular, link to healthy αS tetramers and associated vesicle lipid changes suggest viable drug targets in Parkinson’s disease.
 |
|
|
Study Question
Women are at lower risk for developing Parkinson’s disease than men and often experience milder symptoms, but underlying mechanisms remain unknown. Silke Nuber and colleagues hypothesized female sex may benefit the αS protein homeostasis by supporting the healthy assembly of αS to tetramers, given the necessity of the tetrameric form for normal neuronal function in-vivo.
How This Research Advances What We Know
Initially discovered by the Selkoe laboratory and validated by more than 10 labs, αS can exist in cells in two forms: a stable, helically folded tetramer (T) that resists aggregation, and free monomers (M). Relevant for disease, familial (f)PD-causing αS mutations decrease the physiological T:M ratio.
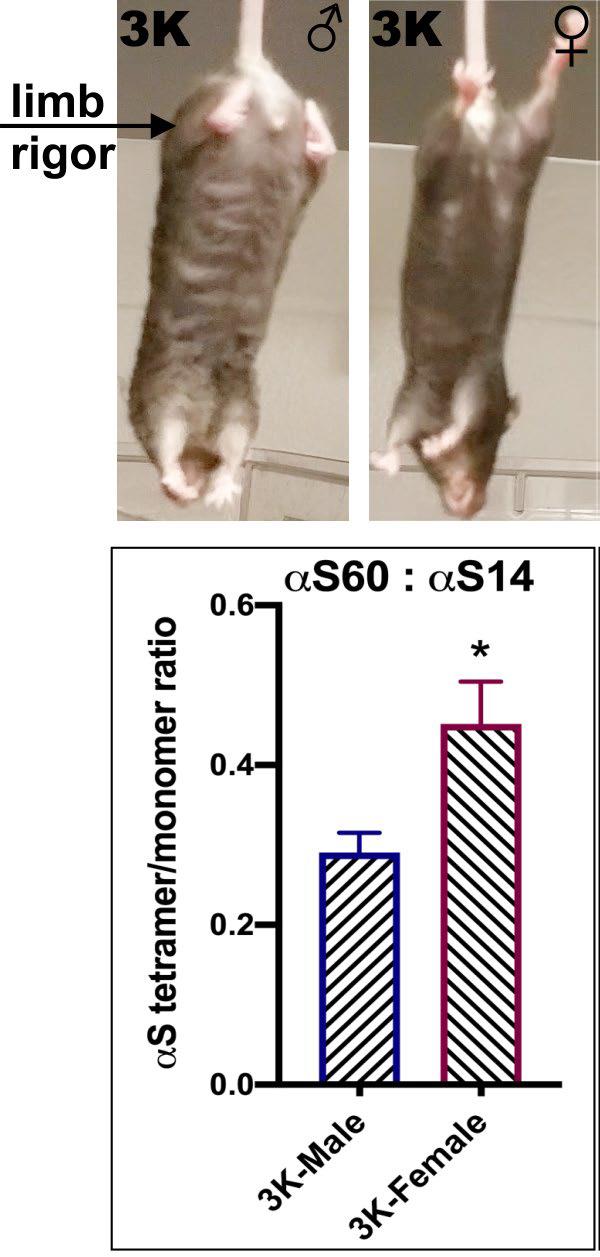
In recent collaboration, Nuber and Selkoe developed fPD-like E-K (“3K”) αS transgenic mice based on destabilizing tetramers, producing a progressive motor-syndrome more robust in male than female mice and the neuropathological changes resemble PD down to the ultrastructural level.
In accord, the insertion of tetramer-abrogating αS mutations slowed kinetics of exocytotic events in cell culture, suggesting that tetramers participate in a normal function of αS in vesicle trafficking. At cell somata, pathologic vesicle aggregation may hamper αS clearance by autophagy, progressing to Lewy body (LB)-type lesions. Additional studies consolidate the relationship between GBA mutations, a major PD risk factor, and male sex predominance in LB pathology of DLB patients.
Importantly, GBA deficiency leads to a decrease in the normal αS T:M ratio in dysfunctional lysosomes, and genetic or pharmacological restoration of normal glucosylceramide levels corrects the αS homeostasis in human cell culture. However, little experimental work has addressed how sex factors in.
Experimental Design or Methodology
In the new work and with support from the Women’s Brain Initiative and NIH, Molly Rajsombath together with Nuber and colleagues analyzed sex as a variable and treated symptomatic 3K mice with a molecule called DHED, designed to convert to the female hormone estrogen solely within the brain. The researchers injected DHED to 3K mice that had overt motor disabilities. Estradiol level and estrogen-regulated gene expression was analyzed to validate treatment effects.
To test for PD-relevant neuropathologies, authors used intact-cell crosslinking and sequential extractions to capture both soluble αS tetramers and insoluble aggregated forms in brains. The consequences of the monomer-tetramer shift were evaluated by measuring vesicle and fiber integrity in brain regions critical for motor coordination, and by assessing the motor performance prior and after 3 months of daily DHED application to symptomatic 3K αS tg mice.
The results were compared to level of age-matched, relatively mildly affected female 3K αS tg mice, as well as motorically normal male and female human wild-type αS tg mice.
Results
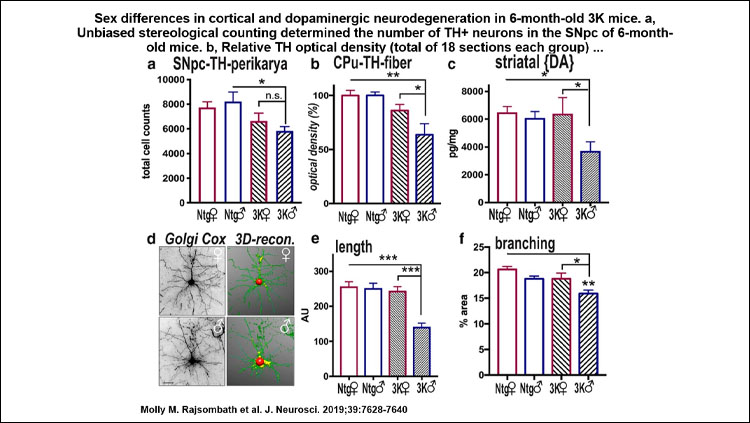
In male 3K αS tg mice, the motor phenotype became apparent at approximately 10 weeks and increased to age 6 months, paralleled by PD-like neuropathology, whereas 3K αS tg females showed a significant delay in onset of the gait impairments. At 6 months, this beneficial phenotypic effect in 3K females was associated with a higher αS T:M ratio and less decrease in neurite fiber length and quantities.
DHED treatment of symptomatic 3K mice significantly increased the αS T:M ratio toward the level detected in motorically normal WT αS expressing mice. This improved tetramer formation was paralleled by a finer distribution of vesicle markers, including the estrogen receptor (ERα), which is known to localize to axonal terminals and dendritic spines, and an increased association between the lysosome-associated membrane protein 1 (LAMP-1), with the autophagy marker LC-3, necessary for normal protein- and lipid-metabolism.
The 3K mouse motor severity correlated with lipid rich-inclusions and SCD1 expression level, the latter a key enzyme for lipid desaturation that has been shown to regulate αS T:M equilibrium and mediate PD-like cytotoxicity, likely due to an adverse αS-lipid interplay. Further, female sex and DHED treatment increased the abundance of the dopaminergic markers tyrosine hydroxylase and VMAT-2 at dopaminergic terminals, in parallel with benefits in motor performance of the 3K mice.
Interpretation
Consistent with a protective role of estrogen against dopaminergic cell loss, female sex and brain estradiol elevation improved the physiological αS T:M ratio in neurons with increased fiber densities and to benefit the PD-like motor deficits. Thus, 3K females are relatively resistant to equivalent excess aS monomers, likely being a result of preserved vesicle flux, which is further paralleled by increased neurite fiber densities and neuronal function.
Given the role of normal lipid homeostasis for sustaining healthy αS tetramers (also regulated by the lipid enzyme GBA1), estrogen may enhance the levels of lipids that support the physiological αS T:M ratio. Together the results support the notion that PD-like motor syndrome and brain pathology in 3K mice is impacted by upstream αS formation to tetramers.
The Nuber Lab is now studying estrogen-signaling pathways that appear to link female sex, and estrogen in particular, with the underlying αS biology of PD, and, together with colleagues, is targeting lipid-modulators as drug targets that could be translated into therapies for patients.
Visit JNeurosci to read the original article and explore other content. Read other summaries of eNeuro and JNeurosci papers in the Neuronline collection SfN Journals: Research Article Summaries.
Female Sex and Brain-Selective Estrogen Benefit α-Synuclein Tetramerization and the PD-like Motor Syndrome in 3K Transgenic Mice. Molly M. Rajsombath, Alice Y. Nam, Maria Ericsson, and Silke Nuber. Journal of Neuroscience 18 September 2019, 39 (38) 7628-7640; DOI: 10.1523/JNEUROSCI.0313-19.2019






