Dynamic Cortical Neural Activity Predicts Self-Guided Working Memory in Rats
Material below summarizes the article The Rat Medial Prefrontal Cortex Exhibits Flexible Neural Activity States During the Performance of an Odor Span Task, published on March 4, 2019, in eNeuro and authored by Emanuela De Falco, Lei An, Ninglei Sun, Andrew J. Roebuck, Quentin Greba, Christopher C. Lapish, and John G. Howland.
Highlights
- Abrupt changes in neural activity patterns observed while rats performed a self-guided working memory task signaled the transition between task epochs and predicted performance on the task.
- Observed neural correlates of a “go” signal might be critical for signaling the transition from “explore” to “exploit” in foraging behaviors.
- Ongoing research will investigate whether the diverse patterns of neural activity observed are altered in brain disorders such as schizophrenia.
 |
|
|
Study Question
We investigated the role of rat medial prefrontal cortex (mPFC) neurons during the execution of a self-guided working memory task. In particular, we asked:
1) How are distinct aspects of the task encoded by the population activity?
2) How is performance of the task related to changes in this neural activity?
How This Research Advances What We Know
Working memory (WM) refers to the ability to hold and manipulate information in the brain during a delay for future use. WM requires several cognitive functions, such as planning, executive control, task monitoring, and memory.
Prior work employing neural recordings has found that optimal WM performance is accompanied by populations of mPFC neurons tracking the various task requirements (e.g., epochs and rules). Notably, persistent elevated activity has been observed in mPFC neurons during the delay epoch of working memory tasks.
This persistent activity was initially considered evidence the mPFC acted as a buffer to temporarily retain information. This view has been recently challenged by studies suggesting the mPFC does not directly store memory information but rather is important for directing cognitive resources and attention toward the relevant neural circuits designated to maintain memory representations.
In this framework, our study aimed to further characterize the contribution of rodent mPFC to WM by employing a well-established measure of WM: the odor span task (OST). The OST is a WM task for rodents that closely resembles human tasks that assess memory span. Performance of the OST depends on a distributed neural circuit including mPFC and dorsomedial striatum. However, to date, no studies have assessed the patterns of neural activity underlying OST performance.
Experimental Design or Methodology
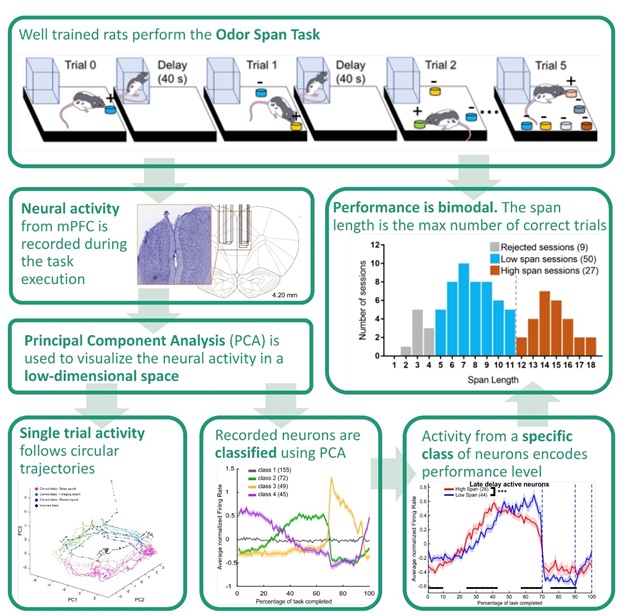
The OST consists of successive trials separated by 40-second delays. In each trial, the rat has to identify a novel odor and dig in a corresponding bowl to receive a food reward. In order to identify the novel scent, the animal must retain a memory of all previously encountered scents. Performance is quantified as the maximum number of familiar scents correctly retained in a session. There are three distinct epochs during each trial: delay, foraging, and reward/error.
In this study, well-trained rats were implanted with mPFC electrodes, and we recorded and analyzed the activity of 382 neurons. Measures of neural firing were assessed across this population to determine the computations mPFC might contribute to this behavior.
More specifically, we used linear statistical approaches to characterize the patterns of activity that populations of mPFC neurons exhibit when this task is performed optimally versus sub-optimally. We used a dimensionality reduction technique, principal component analysis (PCA), to identify the most prominent patterns of activity in the neural population and to classify neurons based on their specific activity profiles.
To make sure the animals relied solely on odor to guide their behavior, we randomized the location of bowls at each trial and minimized the variability of other external factors. Post-hoc analyses showed that behavior was uniform across different performance levels and consistent with a probabilistic distribution, and that performance was uniform across animals.
Results
Behavioral analyses revealed the distribution of task performance was bimodal with a local minimum of 12 odors remembered per session. Therefore, we classified sessions according as “low span” (less than 12 odors remembered) and “high span” (12 or more odors remembered).
When we analyzed neural activity during the delay, we observed neural activity in the mPFC was different between low and high span sessions.
Using PCA, we identified the main neural activity patterns (profiles of activity common to several neurons). Distinct neural populations emerged whose firing rates changed together throughout the different task epochs (e.g., foraging-active neurons, delay-active neurons), with abrupt remapping of neural activity signaling the transition between task epochs.
Using the PCA results, we identified a subpopulation of neurons whose activity increased substantially during the second half of the delay epoch and then decreased sharply at the beginning of the foraging epoch. We found the activity of this specific class of neuron was the best predictor of task performance.
Specifically, in the high span sessions, such increases started earlier and decreased more gradually toward the end of the delay compared with the low span sessions. No differences between firing rates in the low and high span sessions were observed in the remaining classes of neurons.
During foraging, neural activity profiles associated with the approach to novel and familiar odor diverged. In particular, we observed pronounced changes in population activity upon approach to novel odor, while activity patterns upon approach to familiar odor were weaker.
The neural population activity was consistent across trials. When reduced to the first three principle components, neural activity patterns exhibited orbits through state spaces that were highly consistent across all correct trials. Interestingly, neural activity patterns diverged following an error, possibly representing the encoding of an error signal in some mPFC neurons.
Interpretation
The presence of a subpopulation of late delay-active neurons is particularly interesting. Given our neural recordings were acquired from well-trained rats that likely anticipated the end of the delay, the activity of this population may reflect preparation for foraging. The significant divergence of activity patterns on low and high span sessions could be indicative of the readiness of the neural population to transition from delay to foraging epochs, with smoother transitions facilitating the maintenance of information across epochs.
The strong modulation of neural activity upon approach to novel odors could be interpreted as a novelty signal triggering the dig. On the other hand, the lack of evidence for a familiarity signal inhibition the digging was surprising given reports of deficits in response inhibition following lesions of the mPFC.
Collectively, our data highlight the rich and evolving dynamics in the mPFC that emerge throughout the performance of the OST, indicating a broad and diverse involvement of this brain area in the task. Given impairments in WM capacity are a central cognitive symptom of numerous brain disorders, future directions could involve using this task to model WM deficits in rodents and develop novel treatment approaches.
Visit eNeuro to read the original article and explore other content. Read other summaries of eNeuro and JNeurosci papers in the Neuronline collection SfN Journals: Research Article Summaries.
The Rat Medial Prefrontal Cortex Exhibits Flexible Neural Activity States during the Performance of an Odor Span Task. Emanuela De Falco, Lei An, Ninglei Sun, Andrew J. Roebuck, Quentin Greba, Christopher C. Lapish, and John G. Howland. eNeuro March 2019, 6 (2) 0424–18.2019; DOI: 10.1523/ENEURO.0424-18.2019







