Axon-Myelin Pathology Opens Therapeutic Window for Traumatic Brain Injury
Material below summarizes the article Experimental Traumatic Brain Injury Identifies Distinct Early and Late Phase Axonal Conduction Deficits of White Matter Pathophysiology, and Reveals Intervening Recovery, published on October 10, 2018, in JNeurosci and authored by Christina M. Marion, Kryslaine L. Radomski, Nathan P. Cramer, Zygmunt Galdzicki, and Regina C. Armstrong.
Traumatic brain injury (TBI) is a major global health concern. Patients most commonly experience mild TBI and recover, but some patients face a lifetime of persistent symptoms.
TBI can slow transmission of signals within neuronal circuits. This can contribute to diverse functional deficits from early through late stages after TBI. Experimental TBI models provide a means to assess changes in neuronal circuits that may underlie these functional deficits.
In humans and mice, white matter tracts traversing the brain contain long axons especially vulnerable to TBI. TBI causes a characteristic pattern in which degenerating axons are dispersed among many intact axons in white matter tracts.
After modeling experimental TBI in mice, we have shown surviving axons may have myelin sheath damage, or demyelination. Myelin normally wraps around axons to ensure rapid, reliable action potential conduction. Myelin also serves to maintain and protect axons.
Given the role of myelin in aiding signal conduction along axons, we suspected TBI disruption of axon-myelin interactions could contribute to slowed signal conduction in white matter tracts. We used an experimental model of TBI in mice to examine the corpus callosum, which is the largest white matter tract in the mouse brain and a common site of axon damage after TBI in humans.
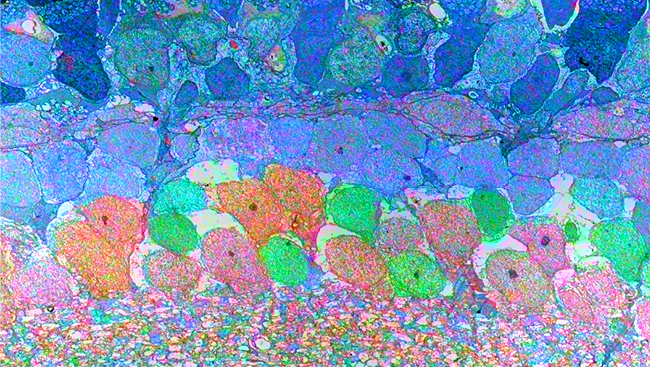
Our collaboration brought together diverse approaches, including the use of fluorescent reporter mouse lines, electrophysiology, electron microscopy, imaging of optically transparent brains using the CLARITY method of tissue clearing, and high-resolution confocal imaging to assess axon-myelin interactions after TBI.
CLARITY imaging of Thy1-YFP mice showed damage in fluorescently labeled axons in the corpus callosum under the TBI impact site. Electrophysiological recordings of signal conduction in corpus callosum axons showed action potential velocity was indeed slower along myelinated axons early (on day three) after TBI.
What structural changes occur in the axon-myelin interactions that contribute to this slowing? We used electron microscopy for ultra-high resolution imaging of axon and myelin structure to identify axons with abnormal paranodes, where the myelin attaches to the axon.
The molecular organization of normal paranodes is well-characterized. The paranode region includes anchor proteins that attach the ends of each myelin sheath segment to the axon. The myelin attached at paranodes leaves a gap, called the node of Ranvier, where sodium channels are clustered to enable propagation of the action potential.
To quantify paranode abnormalities, we turned to immunolabeling and confocal imaging of molecular markers of paranode-node-paranode complexes in the corpus callosum.
The most notable finding was an increase in the number of heminodes after TBI. A heminode is defined as a node flanked by a single paranode on just one side, while the opposite side is missing the paranode anchor proteins required for myelination.
Even minor alterations to myelin attachment and paranode structure can impair signaling, so it is likely these abnormal structures contribute to the slowed conduction observed after TBI.
Having demonstrated slowed conduction velocity and corresponding white matter pathology early after TBI, we considered how signal conduction changes may have an impact at later stages after injury.
Between three days and two weeks after TBI, we observed an increase in the ability of axons to propagate action potentials across the corpus callosum. As degenerated axons likely cannot regrow through this region, this recovery is consistent with potential myelin repair, or remyelination, of intact axons.
To explore this possibility, we used a genetic method to fluorescently label oligodendrocyte progenitor cells to track their maturation into myelin-forming oligodendrocytes.
Surprisingly, the progenitor and mature oligodendrocyte populations were not altered after TBI. Yet interestingly, at four weeks after injury, new myelin was present in all animals, but the TBI mice had synthesized more new myelin than the sham counterparts.
Importantly, the new myelin membranes often extended to paranodes, which suggests functional myelin attachment to axons. The partial recovery in axon signal conduction detected by electrophysiology, together with increased myelin membrane synthesis, indicates recovery after TBI may involve remyelination of surviving axons.
Unfortunately, not all good things last. Electrophysiology at a longer time point of six weeks showed a dramatic loss of axons conducting signal through the corpus callosum. Furthermore, when we lengthened the survival time out to eight weeks, the TBI mice showed atrophy of the corpus callosum. The overall width and area of the corpus callosum were significantly decreased in the TBI mice compared to in the non-injured adult mice.
This time course reveals an interesting progression of myelinated axon conduction deficits and axon-myelin pathology after TBI. TBI produces early conduction velocity deficits and abnormal axon-myelin interactions at paranodes. This damage is followed by an intervening period of remyelination and functional recovery. In the late phase, however, loss of conducting axons is followed by overall atrophy of the corpus callosum.
Further time course studies using magnetic resonance imaging (MRI) in experimental TBI could determine whether these axon-myelin changes correlate with neuroimaging changes, similar to reports of reduced white matter integrity and corpus callosum atrophy in MRI studies of TBI patients at chronic stages.
These findings elucidate the pathophysiological underpinning of diverse symptom expression in TBI patients.
Overall, the myelinated axon conduction deficits and axon-myelin pathology implicate white matter injury in impaired information processing at early and late phases after TBI. Within this time course, evidence of remyelination and partial axon conduction recovery reveals a potential therapeutic window. This axon-myelin pathology opens opportunities for therapeutic targets and reveals a recovery period that may be a critical time to intervene to improve long-term outcomes.
Visit JNeurosci to read the original article and explore other content. Read other summaries of JNeurosci and eNeuro papers in the Neuronline collection SfN Journals: Research Article Summaries.
Experimental Traumatic Brain Injury Identifies Distinct Early and Late Phase Axonal Conduction Deficits of White Matter Pathophysiology, and Reveals Intervening Recovery. Christina M. Marion, Kryslaine L. Radomski, Nathan P. Cramer, Zygmunt Galdzicki, Regina C. Armstrong. JNeurosci Oct 2018 38 (41) 8723-8736; DOI: https://doi.org/10.1523/JNEUROSCI.0819-18.2018